Separating sulfur
Published by Callum O'Reilly,
Senior Editor
Hydrocarbon Engineering,
Liquid redox is a process used to remove hydrogen sulfide (H2S) from sour natural gas and other gas streams by converting it to solid elemental sulfur for sale or disposal. It is based on a reduction-oxidation reaction carried out in an aqueous solution and catalysed by iron which is held in solution by organic chelating agents. The spent solution is then circulated to an oxidiser to be regenerated with air and reused. This process forms solid sulfur particles that can be easily filtered from the solution.
As shown in Figure 1, the typical applications are streams where the total amount of sulfur removed is greater than 0.5 tpd and less than 20 tpd. Liquid redox processes are not usually economical for streams that contain very small amounts of sulfur, where the total amount is less than several hundred pounds per day.
The niche for liquid redox processes can be found between two extremes – sour gas streams with high concentrations of H2S and a constant throughput near the design point, where the Claus process is utilised, and those with very dilute H2S concentrations, where non-regenerable scavengers are applied in towers or by direct injection.
Liquid redox can process virtually any kind of gas stream and, as a result, the technology has been applied in a wide variety of applications to achieve 99.9%+ H2S removal in a single stage.
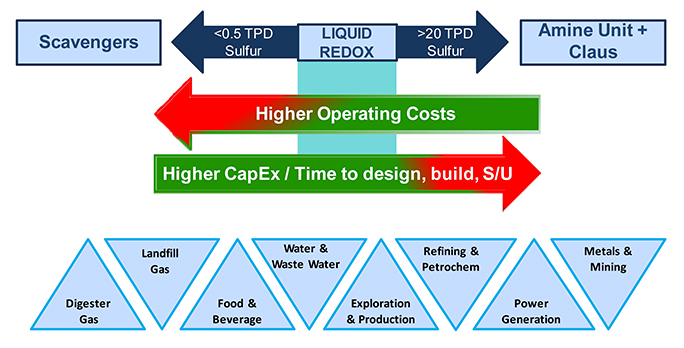
Figure 1. The application niche for liquid redox technology.
How it works
H2S contacts the liquid redox solution inside the absorber and is converted to elemental sulfur and water. The solution is then routed to the oxidiser where the catalyst is regenerated using air. Regenerated solution is pumped back to the absorber. A slip stream of solution from the oxidiser is routed to a sulfur filter to produce sulfur cake. Depending upon the type of sulfur filter chosen, a settler may be employed to concentrate the solution into a slurry with higher solids content. This can improve the operation of the sulfur filter. The redox process utilises an aqueous solution of iron, whose solubility in water and catalytic performance is enhanced by a proprietary blend of chemicals. The H2S is converted to elemental sulfur by redox chemistry according to the following overall reaction:
Direct oxidation reaction: H2S + ½ O2 ? H2O + S°
Equation 1 is the same (overall) as the Claus reaction, but the mechanisms are very different. The redox reaction is carried out in separate sections of the process as summarised in equations 2 and 3:
Absorber: H2S + 2Fe+++ ? S° + 2Fe++ + 2H+
Oxidiser: ½ O2 + H2O + 2Fe++ ? 2OH– + 2Fe+++
The exothermic absorber reaction represents the oxidation of H2S to elemental sulfur and the accompanying reduction of the ferric iron state (active) to the ferrous iron state (inactive). This reaction is irreversible and not equilibrium dependent. The oxidiser reaction (also exothermic) represents the oxidation of the ferrous iron back to the ferric iron state.
All reactions take place in solution at near ambient temperature, making the process inherently safe. The working solution operates at a pH between 8 and 9. NaOH or KOH is used to control the pH.
There are no known contaminants that will completely inhibit the reaction of H2S to elemental sulfur. However, it is undesirable to contaminate the solution with liquid hydrocarbons or amine, both of which can cause foaming that leads to operational problems...
Written by David Jackson, Merichem Process Technologies, USA.
This article was originally published in the December 2021 issue of Hydrocarbon Engineering magazine. To read the full article, sign in here or register for a free trial subscription.
Read the article online at: https://www.hydrocarbonengineering.com/special-reports/29122021/separating-sulfur/
You might also like
The Hydrocarbon Engineering Podcast - Education and training for every phase of the insulating system design process
In this episode of the Hydrocarbon Engineering Podcast, Brandon Stambaugh, Owens Corning Director for Technical Services, joins us to discuss engineers’ demand for education and training to support the critical phases that affect the performance and longevity of insulating systems.
Tune in to the Hydrocarbon Engineering Podcast on your favourite podcast app today.

