Increased reliability in butadiene production
Published by Pippa Luck,
Editorial Assistant
Hydrocarbon Engineering,
In recent years, the chemical industry has made significant progress to reduce costs and improve productivity by increasing the time between plant turnarounds. Improved fouling control has been an important contributor to this progress, and also reduces energy costs, maintenance costs, and carbon emissions. In butadiene production, fouling control in the extraction section of the butadiene process is one of the most important challenges limiting run length and reliability. This article describes the chemistry of fouling in this section and the application of ACtify® technology for improved fouling control in butadiene extraction.
ACtify is the brand name used for the patented polymer inhibitor technology of Dorf Ketal. It has been discovered that certain tertiary amines formulated with existing inhibitor technologies will dramatically improve the speed and efficiency of the reactions inhibiting polymerisation.1 The concept is to ‘activate’ the inhibitors to deliver faster kinetics in polymer control reactions.
Success with this technology in styrene is well documented.2,3 In the butadiene process, the improved speed of inhibition reduces the rate of butadiene polymerisation and inhibits formation of butadiene dimer. Previously, it was thought that the speed of dimer formation was too fast for any inhibitor to stop. For example, the American Chemistry Council butadiene product stewardship manual, last published in 2019, states: “at present, there is no known inhibitor for the butadiene dimer reaction”.4 Developing the first ever dimer inhibitor is testimony to the impact of ACtify technology on the speed and efficiency of inhibitor chemistries.
Fouling in the extraction section due to polymeriation is caused by a combination of factors, including monomer concentration, iron, oxygen, presence of C5’s, low velocities, temperature, and residence time. Solvent degradation and the use of antifoams increase the complexity of controlling the overall rate of fouling. Fouling is a non-linear process with potential for unexpected spikes in the rate of fouling. The lack of predictability increases the risk of an unexpected interruption in production and all costs involved with an unplanned shutdown.
Improved monitoring techniques offer the butadiene producer a substantial improvement in fouling control. This offers better predictability of planned outages, improving productivity and reducing energy and maintenance costs.
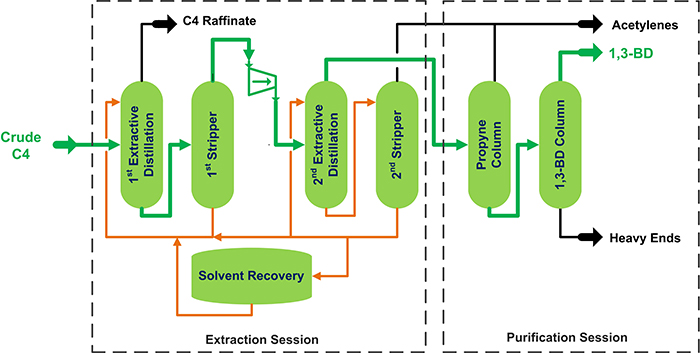
Butadiene production process
Along with ethylene and propylene, 1,3-butadiene (1,3-BD) is one of the most valuable monomers produced through the steam cracking of hydrocarbons. Once fractionated in the debutaniser of the olefins plant, the C4 cut containing a mixture of butanes, butenes and butadienes (commonly called crude C4’s), is the feedstock to the butadiene unit (see Figure 1). The concentration of 1,3-BD in the crude C4 stream is typically on the order of 40 – 50%. The balance is other C4’s along with some C3 and C5 contaminants. Due to the close boiling points and relative volatilities of butadiene and other C4 compounds, they cannot be commercially separated through a simple distillation. Selective solvents are used to extract the 1,3-BD from the rest of the C4’s.
There are several commercial designs of butadiene units using solvents such as N-methylpyrrolidone (NMP), dimethylformamide (DMF), or acetonitrile (ACN), to extract the 1,3-BD from the crude C4 mixture. After extraction, the 1,3-BD is removed from the solvent and distilled to a high purity product.
Figure 1. Example of butadiene unit.
In the first extractive distillation section, the butanes and butenes are removed in the overhead, shown in Figure 1 as C4 raffinate. The overhead stream of the second extractive distillation column concentrates the 1,3-BD for downstream purification. The purification section removes acetylenes and other minor contaminants, increasing the 1,3-BD concentration from 95 – 98% to at least 99.5% for the final product.
Solvent is recovered and reused. Part of the solvent is continuously sent to a recovery unit, where water and heavies (degradation products, polymers, and inorganics) are removed to meet the solvent quality specification.
Extraction section fouling control
It is well known that both temperature and peroxide initiated mechanisms contribute to the polymerisation tendency of 1,3-BD. Temperature initiated polymerisation is the primary mechanism for polymers formed in the extraction section (Figure 2a.) In the purification section, polymer formation is primarily peroxide initiated (Figure 2b – also known as popcorn polymer). Considering the temperatures and concentrations of 1,3-BD, the butadiene unit is prone to fouling in all sections. The fouling seen in the extraction section is usually not related exclusively to the polybutadiene polymer, but also due to solvent degradation, iron, C5 and oxygen contamination, and antifoam injection. Other factors that complicate fouling control include periods of low load conditions or efforts to push plant capacity above design by processing C4 feedstocks from other ethylene plants. The combination of mechanisms makes fouling control in the solvent loop challenging. In many cases, treatments recommended by the licensors are not enough to meet operating goals that can exceed original design parameters, such as increased run-length or throughput.
Figure 2. Butadiene polymers from extraction (left) and purification areas (right).
Chemistry of polymer control
The first reaction of two butadiene monomers in the solvent loop is the formation of butadiene dimer. It has long been theorised that dimer formation is partly the result of a free-radical mechanism.5 High temperatures in the solvent loop increase the rate of free radical formation. The speed of reaction of ACtify chemistry is fast enough to inhibit the free-radical mechanism of dimer formation. Slowing polymer formation earlier in the polymerisation process gives the technology an advantage over other options, as the concentration of free radicals are lower, reducing inhibitor demand. Faster terminating reactions reduces the chemical required to control polymer formation and the lowers the polymer molecular weight, reducing fouling tendency.
4-Hydroxy-Tempo (4HT) is often used for free radical polymer inhibition in ethylene, butadiene, and styrene production. As shown in Figure 3, the ACtify version of 4HT works better at less than half the dosage. The faster speed of reaction with the free-radicals provided by ACtify lowers the polymerisation rate, reducing chemical demand. Polymers formed are terminated at lower molecular weights, reducing risk of deposition.
Figure 3. Polymerisation test with 4HT (Butadiene - 248F/120°C – 4 hrs) – Activated with ACtify and non-activated1 (Patent US9598333).
An additional benefit of this technology is the potential to reduce NOX formation in the ethylene furnace in the event there is a need to reprocess C4 streams from the butadiene unit to the ethylene furnace.6
Case studies document how ACtify technology has been able to increase run-length, reduce energy costs, and reduce cleaning frequency of strainers and filters.
Figure 4. Solvent stripper flow corrected DP for two runs.
Case study 1
This 100 000 tpy butadiene unit was facing severe fouling problems in the stripper column, where the C4’s are removed from the solvent. The competitor chemical treatment was unable to control the rate of increase in the column Delta P (Figure 4). Prior to implementation of the Dorf Ketal programme, the run lengths were up to 24 months, requiring throughput reduction during the run and unscheduled shutdowns.
With the use of ACtify technology, the plant has been able to achieve more than 30 months of run and increase the efficiency of the heat recovery system, reducing energy costs. Figure 5 shows comparative heat transfer performance.
Figure 5. Recovery heat exchanger performance.
Case study 2
This 120 000 tpy butadiene unit was operating with licensor recommended anti-foam and solvent corrosion inhibitor. Two years into the run, the plant began to see an increase in the strainer cleaning frequency and a significant reduction in heat transfer efficiency. Analysis of the foulant indicated the presence of butadiene polymers in the extraction section. Since the implementation of ACtify technology, the polymer formation and accumulation has dramatically reduced. Figure 6 shows how the time between strainer cleaning has increased.
The improvement in polymer control has stabilised the performance of the recovery heat exchanger and stripper reboiler, a clear indication that fouling is under control.
Figure 6. Extraction section pump’s strainer cleaning frequency.
Conclusion
There is an increasing need for improved run-length and reliability of the butadiene section of the olefins complex. Fouling control in the extraction section of the butadiene process is one of the most important challenges limiting butadiene plant run length and reliability. ACtify technology offers a new strategy for improved fouling control in butadiene extraction.
The design of the chemical treatment needs to consider the plant conditions, run-length targets, and contaminants present. As the case histories have shown, fouling control can be achieved. This offers better predictability of planned outages, improving productivity and reducing energy and maintenance costs.
Written by Joice Gorete Boll, Dorf Ketal, Brazil.
References
- SUBRAMANIYAM, M., ‘Additive Composition for control and inhibition of aliphatic monomers, and methods and preparation and use thereof’, US 9598333, United States Patent and Trademark Office, (20 March 2017).
- ONDYAK, J. NOLAND, J. and RAMASWAMY, P. N., ‘Comparing DNBP Replacement Options in Styrene Production’, Hydrocarbon Processing, (November 2017).
- CHOKSI, J. et.al., ‘A Study in Sustainable Styrene,’ Hydrocarbon Engineering, (December 2018). American Chemistry Council, ‘Butadiene Product Stewardship Manual’, (2019).
- ALDEEB, A., ROGERS, W., and MANAAN, M., ‘Evaluation of 1,3, butadiene dimerization and secondary reactions in the presence of oxygen’, Journal of Hazardous Materials, (August 2014).
- SUBRAMANIYAM, M., and KNIS, M, ‘Quantifying the Risk of NOx Accumulation in the Ethylene Cold Box from the Use of Polymerization Inhibitors,’ Paper 41C, Ethylene Producers Conference, (April 2021).
Read the article online at: https://www.hydrocarbonengineering.com/special-reports/14072021/increased-reliability-in-butadiene-production/
You might also like
The Hydrocarbon Engineering Podcast - Education and training for every phase of the insulating system design process
In this episode of the Hydrocarbon Engineering Podcast, Brandon Stambaugh, Owens Corning Director for Technical Services, joins us to discuss engineers’ demand for education and training to support the critical phases that affect the performance and longevity of insulating systems.
Tune in to the Hydrocarbon Engineering Podcast on your favourite podcast app today.