Kurita: Away with the salts
Published by Bella Weetch,
Editorial Assistant
Hydrocarbon Engineering,
Wash water and salts: water on earth is an indispensable basis for the earth's ecosystems and human life. Along with climate change, water scarcity is an important future issue for humanity. Therefore, it is of great importance to use water in industrial processes only in a targeted manner or to significantly reduce the necessary quantities. With the help of the ACF technology presented here, water consumption can be significantly reduced.
Ammonium chloride (NH4Cl) and ammonium bisulfide (NH4HS) are corrosive, as a gas, as a solid, or in solution. Apart from corrosion blockage due to a salt deposition can result in increased delta pressure across the equipment. If deposition happens on tower trays it can harm distillation performance. In petroleum refining plants, wash water is used at many locations to dissolve ammonium salts from metal surfaces or to remove the salts with the wash water. This significantly reduces the risk of salt fouling and corrosion, but unfortunately still does not lead to the desired results in some cases. A good design of the wash water system is necessary for salt removal, but sometimes an uncontrollable discharge temperature still causes problems. If wash water is not sufficient and salts are not completely dissolved, then it can result in a thick and viscous salt solution. If the fluid velocity in condensers is low enough for salts to settle in the tubes, pitting and under-deposit corrosion can occur. Typical problem areas are CDU or FCC distillation column head sections, feed effluent exchangers of hydrocrackers, hydrotreaters or catalytic reformer reactors, recycle gas compressors, and stabiliser column head sections.
ACF technology
The ACF technology is strong-base chemistry that reacts directly with hydrochloric acid or ammonium salts. It has three very important properties:
- High ammonium salt replacement effect.
- Very low corrosiveness of the formed salts.
- High moisture absorption.
The strong base ACF replaces the weaker base ammonium from its salts and forms liquid ACF salts with neutral pH. Interestingly, there are process plants where regular water washing removes only small amounts of salt deposits. It is assumed, that hydrocarbons prevent direct contact of the wash water with the salts. There are examples where the chloride concentration in the wash water increased rapidly from <10 ppm to >5000 ppm chlorides after the addition of ACF. At the same time, a lot of wash water can be saved, which is a very important factor in many parts of the world to save water and reduce energy costs. Availability of the right quality of wash water is a major challenge for refineries. Wash water may also lead to reduced production and/ or product contamination that increases reprocessing costs.
The ACF technology is used during online cleaning to remove ammonium salts or neutralizing amine hydrochloride (e.g. MEA hydrochloride). When large amounts of ammonium salts need to be dissolved and removed, it is particularly important to provide a sufficiently high amount of ACF during cleaning. In practice, it has proven useful to apply a ratio of 1:5 or 5 parts ACF to 1 part deposited salts as a rule of thumb. For continuous applications as a preventive measure, dosing is usually realised in the low ppm range. Initial cleaning with a high amount of ACF and then continuous dosing of ACF in low amounts ensures no deposition of salts during operation.
Benefits of ACF technology:
- Prevention of salt fouling and corrosion by forming liquid ACF salts. These salts have a neutral pH and very low corrosion potential.
- Removal of already formed ammonium salt deposits during operation.
- Immediate reaction with very corrosive hydrochloric acid and ammonium salts.
- Formed ACF salts can be easily removed with wash water or sour water. Liquid ACF salts are hygroscopic and have a very low corrosion potential.
- Ensures a long lifetime of the process equipment.
- Reduces the need for cleaning operations.
Case study
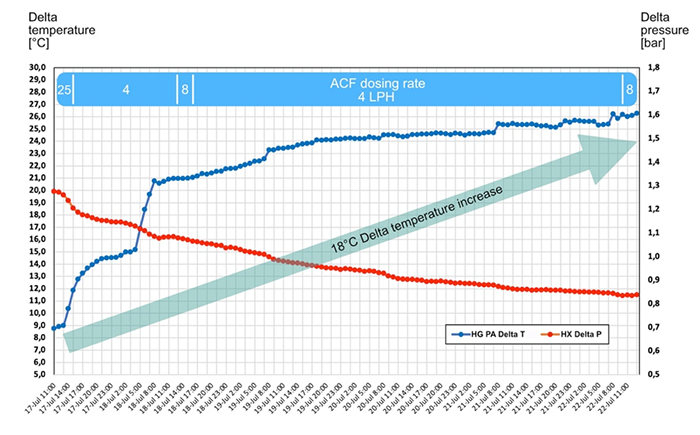
A refinery observed ammonium salt fouling in the Residue FCC (RFCC) main fractionator column upper trays, overhead system and depropaniser reboiler. Up to 70 ppm chlorides are regularly measured in the overhead sour water. Every two months a 2 - 8 hr online water wash with ‘out of spec production’ must be carried out, where up to 13850 ppm of deposited chlorides are dissolved and removed with the wash water. After water washing a 10 - 15 °C heat recovery on the hot side of the circulating heavy gasoline was observed. The graph below shows the trial results, where an ACF additive was continuously added to the system. The depropaniser bottom temperature and reflux ratio were increased, while LCO flow rate to the depropaniser reboiler was reduced and delta temperature increased to 18 °C.
Is your refinery facing a problem of ammonium chloride/bisulfide corrosion and fouling? Is high wash water requirement increasing your operational cost and reducing productivity? Consider using Kurita ACF Technology online that can remove salt deposits with reduced to no wash water requirement.
Contact Kurita for further information.
Read the article online at: https://www.hydrocarbonengineering.com/special-reports/08072021/kurita-away-with-the-salts/
You might also like
The Hydrocarbon Engineering Podcast - Education and training for every phase of the insulating system design process
In this episode of the Hydrocarbon Engineering Podcast, Brandon Stambaugh, Owens Corning Director for Technical Services, joins us to discuss engineers’ demand for education and training to support the critical phases that affect the performance and longevity of insulating systems.
Tune in to the Hydrocarbon Engineering Podcast on your favourite podcast app today.